Figure 1
High Selectivity Methane Coupling at High Pressure Using Novel, Defect/Disordered Rare Earth Oxycarbonate Based Catalyst Systems
M. M. Bhasin, R. D. Cantrell, A. Ghenciu, K. D. Campbell, D. M. Minahan,
A. D. Westwood, K. A. Nielsen
The Dow Chemical Company
South Charleston, West Virginia 25303
Keywords: Oxidative Coupling of Methane (OCM), rare earth oxycarbonates, high pressure, lower temperature, transition metal and other metal oxides additives
1. Introduction
Methane is an attractive raw material for producing ethylene and propylene because it is widely available and inexpensive compared to the natural gas liquids (ethane, propane, butane and higher hydrocarbons). However, supply of natural gas liquids have not kept pace with the demand for ethylene and propylene, hence more costly cracking processes that use naphtha from petroleum are being commercialized. Therefore, development of economical processes for manufacturing olefins and other hydrocarbons from methane is highly desirable.
Oxidative coupling of methane was first discovered by Keller and Bhasin in the 1970s and first reported in 1982(1). The field of oxidative coupling exploded exponentially in the 1980s and early 90s(2). A vast majority of these 1500+ publications and patents attempted such oxidative coupling at one-atmosphere pressure and 750-850°C over a variety of catalysts spanning most of the periodic table. Primary focus was on increasing conversion and selectivity or yield of C2+ hydrocarbons at one atmospheric pressure. Few attempts were made to accomplish methane coupling at higher pressures since higher pressures are necessary for a commercially viable process, however, selectivity dropped sharply at pressures of 5+ atmospheres(3).
2. Experimental
Several methods of catalyst preparation will be covered and are described in reference 4. Catalyst of 14/30 mesh size were evaluated in a tubular microreactor at typically 125 psig (sometimes 125-250 psig) using methane and oxygen in a ratio of 9:1 at a Gas Hourly Space Velocity of 30,000 hr-1. Reaction temperature was raised to 450°C and then raised repeatedly in 50°C increment up to 700°C. Details of various characterization techniques used will be described. These techniques are: XPS, HRTEM and EELS as well as more routine physical and chemical analyses.
3. Results and Discussion
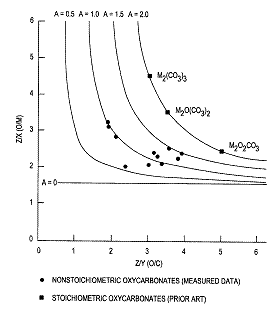
A very important challenge in coupling methane at higher pressures of 10-20 atmospheres is the precipitous loss in selectivity over all prior art catalyst compositions. According to published reports(3), selectivity to C2+ products decreases by 3-4% per one atmosphere increase in pressure. Thus, a catalyst having 80% selectivity at 1 atm. will only be 50-40% selective at 10 atm. and even lower selectivity at >10 atm. pressure. These low level of selectivities are unacceptable for an economically attractive process. An exhaustive investigation recently revealed several series of unique and novel catalyst systems that give 70+% selectivity to C2+ products at pressures of 10+ atm(4) and at temperatures of 450-600°C. These catalysts are broadly classified as non-stoichiometric, rare earth oxycarbonates of the formula MxCyOz having a disordered and/or defect structure, wherein M is at least one rare earth element selected from the group consisting of La, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er and Tm; x = 2; z = 3 + Ay; A is less than about 1.8, and y is the number of carbon atoms in the oxycarbonates (Figure 1). The extent of disorder/defect were
Figure 1 |
|
measured by determining the stoichiometry of the rare earth oxycarbonates by Electron Energy Loss spectroscopy on a scanning transmission electron microscope. These novel, rare earth oxycarbonates are further characterized by high resolution electron microscopy as having swiss-cheese like appearance – thus highlighting the disordered/defect, non-stoichiometric nature (Figure 2). Such atomic level characterization will be shown to correlate with catalyst selectivity and activity. On the other hand, stoichiometric oxycarbonates are essentially inactive under such conditions up to ~450-650°C at ~10 atm. (Figure 3). These novel catalysts may further contain at least one co-catalyst selected from the elements, V, Nb, Ta, Cr, Mo, W, Mn, Re, Fe, Co, Ni, Cu, Zn, Sn, Pb, Sb and Bi. Catalyst performance in microreactor tests will be presented including good catalyst stability for 30 days. These novel catalyst systems also exhibit an inverse pressure effect whereby they are less selective at 1 atm as compared to the selectivity at 10 atm.
Figure 2 |
Figure 3 |
|
|
4. Conclusions
Several series of non-stoichiometric, rare earth oxycarbonates based catalyst systems have been discovered that provide, for the first time, 70+% selectivity to C2+ hydrocarbons at 125+ psig pressure and 450-600°C in microreactors that are stable for ~30 days. These novel catalyst systems are characterized as having non-stoichiometric, defect/disordered surface structure – as determined by various atomic level resolution techniques. Major challenges still remain to scale-up of catalyst, improved aging and a viable process scheme.
References
(1) G. E. Keller and M. M. Bhasin, J. Catalysis, 73, 9 (1982).
(2) M. M. Bhasin and K. D. Campbell, “Methane and Alkane Conversion Chemistry,” editors, M. M. Bhasin and D. W. Stocum, Plenum Press, NY, 1995.
(3) (i) C. Cameron, H. Mimoun, S. Bonnaudet and A. Robin, US Patent 4,929,787, May 29, 1990.
(ii) M. Pinabiau-Carlcer, et.al, in “Natural Gas Conversion (1991) ed. A. Holmen et.al, pp. 183-190.
(4) R. D. Cantrell, A. Ghenciu, K. D. Campbell, D. M. Minahan, M. M. Bhasin, A. D. Westwood, K. A. Nielsen, US Patents, 6,403,523B1, June 11, 2002 and 6,576,803B2, June 10, 2003.